
Leptospirosis
A page about leptospirosis in dogs describing causes, clinical signs, diagnosis and prevention.

Introduction
Leptospirosis is currently considered to be the most widespread zoonotic infection in the world. Infected dogs can be a source of infection for people in which it may also cause a potentially life-threatening disease. A critical component of disease control is therefore the prevention of shedding, best achieved by using a vaccine that has demonstrated its ability to do this. More information on this aspect of disease is discussed under the control section.
Aetiology
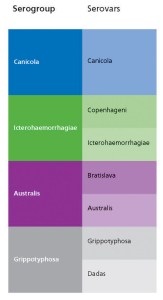
Leptospirosis is caused by microscopic bacteria (Leptospira spp.) belonging to a group called spirochaetes. Different variants of Leptospira spp. are known as serovars. Those that are antigenically similar are grouped together in serogroups. Cross-protection against unrelated serovars is not expected although vaccines containing a certain serovar may sometimes be shown to cross-protect against serovars within the same serogroup provided they are closely related.
Some confusion also arises because serovars and serogroups occasionally share the same name.
By way of clarification the chart opposite shows Serogroup and Serovar classification of significant pathogenic leptospires.
Epidemiology
After ingestion of the bacteria, the leptospires enter the blood stream via the mucous membranes. This is followed by a rapid replication in several tissues such as the kidney, liver and spleen. The bacteria are then excreted via the animal’s urine back into the environment.
Risk Factors
Virtually all dogs are at risk. However, the highest risk activities include:
• Access to still or slow-moving water sources
• Exploring the outdoors
• Contact with known reservoir species
(e.g. wild rodents) – e.g. farm dogs
• Hunting or herding dogs
Transmission
• Chronically infected reservoir host species such as rodents maintain disease in the environment and can shed leptospires for months or even years without showing clinical signs
• Infected animals shed bacteria in their urine and transmission can occur either directly through contact with the infected urine, or indirectly – for example through contact with contaminated water
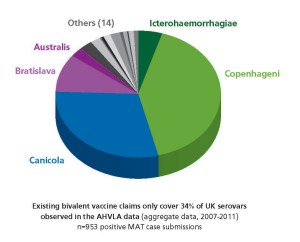
For more than fifty years, the serovars covered by Irish and UK leptospirosis vaccines have remained unchanged: Canicola and Icterohaemorrhagiae. Current vaccines continue to perform well against these serovars. But in the field, things have changed. The epidemiology of leptospirosis has undergone a marked shift. The pie chart shows the UK serovars identified in the most recent MAT-positive clinical submissions to the Animal Health and Veterinary Laboratories Agency (AHVLA) (UK). Unfortunately no Irish data are yet available to draw similar comparisons.
Most notable is the increased seroprevalence of serovars Bratislava and Copenhageni. Together with serovars Canicola and Icterohaemorrhagiae these account for 85% of positive results in the AHVLA data over the most recent 5 years data available (2007-2011).
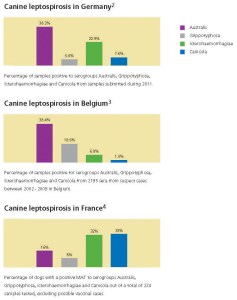
It is increasingly clear that Europe, too, must recognise the need for change. Data from Germany, France and Belgium show that cases of leptospirosis associated with emerging serovars from the serogroups Grippotyphosa and Australis occur at least as frequently as those from Canicola and Icterohaemorrhagiae.
Record numbers of dogs now travel into Europe and back into Ireland and/or the UK. It is therefore important that our vaccines take account of the wider changes being seen in this important zoonotic disease’s epidemiology. Data on the relative prevalence of serovars from selected EU member states are shown below.
Clinical Signs
Early signs can be vague and non-specific, and the disease is likely to be under-diagnosed
• However, if not treated early, the disease may progress to potentially fatal hepatic and/or renal failure. Initially a mild disease, if untreated it can progress rapidly to failure of these organs resulting in more severe clinical signs such as abdominal pain, jaundice, liver damage and even death.
• Even if treated with antibiotics, infected animals may continue to shed the bacteria in their urine

Diagnosis
Diagnosis can be based on:
- Clinical signs – Clinical signs of red urine (haemoglobinuria) are occasionally detected early in disease. This later progresses to jaundice.
- Haematology / serum biochemistry – A blood test may show a severe decrease in the white blood cell numbers and/or damage to the liver and kidneys. These are not specific for leptospirosis however.
- Bacteria may be detected by special microscopy of fresh urine. A PCR is also available for detecting bacteria in various tissues though its validation has been called into question.
- Serology – Further analysis of the blood may also indicate exposure to Leptospirosis (antibodies) if the animal survives long enough to mount an immune response.
- Post mortem examination of the animal can confirm characteristic pathology that can be confirmed by various tests.
Control
A useful approach to control is to avoid risk factors outlined under epidemiology above. Vaccination is probably the most widely used tool in control however.
A recent Veterinary Record paper by leading expert Dr W A Ellis has reviewed the situation regarding control of leptospirosis. In this paper it was stated – “… it is safe to conclude that serovars Icterohaemorrhagiae and Canicola should continue to be included in dog vaccines in Europe. In addition, there is a good case for the inclusion of serovar Bratislava in vaccines, and, in the case of mainland Europe (but not in the UK), for serovar Grippotyphosa to be included as well.”
MSD Animal Health has recently introduced to the Irish market the first canine leptospirosis vaccine to include serovars from all four key serogroups in Ireland, the UK and continental Europe, with specific claims against:
Serovar Copenhageni (Serogroup Icterohaemorrhagiae)
Serovar Canicola (Serogroup Canicola)
Serovar Bratislava (Serogroup Australis)
Serovar Dadas (Serogroup Grippotyphosa)
Typically cross-protection provided by leptospirosis vaccines against specific serovars may be demonstrated by a combination of challenge trials and serological assessment.
Further studies carried out with this vaccine and serovars Icterohaemorrhagiae and Grippotyphosa suggest that the new vaccine is likely to cross-immunise against these serovars too.
This vaccine may only be prescribed by your veterinary practitioner from whom advice must be sought. For further information on disease and vaccination please click here.
