
Lungworm

A page about lungworm (hoose) infection in cattle, its causes, clinical signs, diagnosis, treatment and control
Introduction
Lungworm infection causes a severe and often fatal disease that is commonly caused hoose or husk. Outbreaks continue to rise year on year. The number of cases of lungworm infection has risen dramatically since the 1990s.
The result? Milk yields melt away, fertility drops and calving periods extend. The cost of drugs, labour and veterinary treatment build up. Worst of all, valuable animals die or have to be culled.
Aetiology
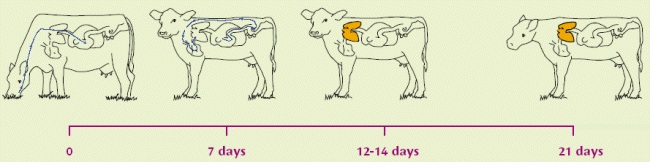
Lungworm infestation is caused by exposure of grazing animals to nematode (roundworm) larvae on pasture. The lifecycle of the lungworm is about 4 weeks long i.e. from the ingestion of larvae to the excretion of infective larvae by the affected animal. In the worst case, within four weeks of ingesting lungworm larvae, the cow or calf can be shedding literally millions of fresh larvae onto the pasture. A faecal output of 1,850,000 larvae per day has been recorded!
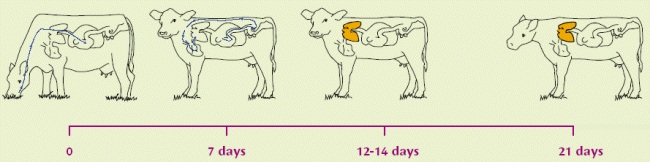
| Infective larvae (L3) eaten from pasture. | Migrating, at 1 week, as L4 out of the intestinal wall via the bloodstream to the lungs. | Becoming L5 (immature adults) within a few days, moving up to upper respiratory tract. L5 can over-winter in carrier hosts. Immunity established/active at this stage | Become mature adults: slender white worms up to 8cm long, living in the lungs. |
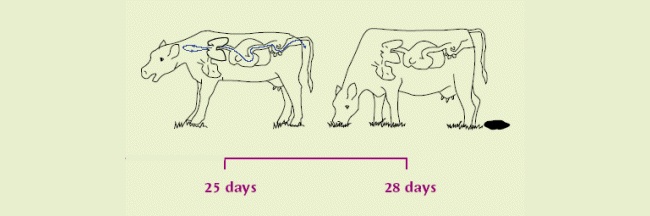
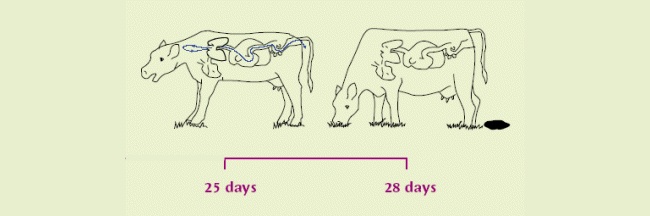
| The infection now becomes patent. Eggs containing L1 are coughed up and swallowed by the same cow, hatching to L2 on the way down and out in faeces. | After 1 week on pasture, become infective L3. Scattered by fungus/rain for over a metre. Thought to over-winter on pasture in some circumstances. |
Epidemiology
The risk of disease depends on the level of challenge which in turn depends on a number of factors as follows:
1). FARM HISTORY – It has been suggested that a farm’s history of lungworm outbreaks is a reliable indicator of the likely degree of future challenge – and it is certainly true that larvae can survive a long time on pasture. However, following extensive analysis of countrywide data it now seems more plausible that the mere presence of cattle predisposes that pasture to lungworm, even if only at very low levels. The parasite and its host have a long-established relationship, and it is only a sudden change in circumstances or immunity status which allows lungworm growth to accelerate to pathological levels.
Where there are cattle, there is likely to be lungworm.
2. HUMIDITY AND RAINFALL
Rain can disperse larvae in contaminated faeces, while warm, moist conditions keep infective larvae alive and encourage fungal growth. Larvae often make use of the fungal spore of Pilobolus to disperse themselves on pasture. Generally, conditions which favour the growth of pasture also favour development of the infective larval stage L3, which is why outbreaks peak in the late summer and early autumn.
A dry season followed by a damp one has always encouraged outbreaks, as this creates a natural immunity gap. This entirely natural situation is one we are now mimicking through our long acting worming policies.
3. GEOGRAPHICAL LOCATION
Thanks to varying climates and cattle densities, some counties are more prone to infection than others.
3. REPLACEMENT STOCK
The introduction of immunologically-naïve replacement stock can severely destabilise a herd’s balance of immunity. In such circumstances a much lower level of existing challenge is sufficient to cause an outbreak.
The introduction of ‘carrier’ animals among replacements is thought to be less of a problem, although it has been recorded.
4. OFF-FARM OR RESERVED PASTURE FOR REPLACEMENT STOCK
A growing trend is to graze dairy replacements on separate pasture, away from the main herd. Frequently this stock is subject to continuous worming, perhaps in both the first and second seasons. When the replacement group finally enters the main herd, they are completely naïve and an outbreak is highly likely.
This situation contrasts strongly with beef suckler herds, where calves are exposed to continuous low level challenge from their immunologically-competent adult companions.
Most vets now agree that the changing pattern of the disease can be attributed to the decline in immunity. Immunity can be achieved by routine vaccination – indeed, the statistics show that if vaccination is suddenly halted, the chance of a future outbreak is 63% – alongside the increasingly unpredictable levels of immunity associated with the use of powerful long acting wormers in the first and/or second seasons at grass.
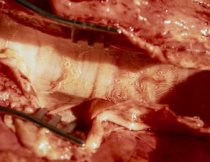
Clinical Signs
Clinical signs mainly involve coughing and increased respiratory rate, and is commonly known as hoose or husk. This can initially be evident only when animals are disturbed but progresses quickly to frequent coughing even in resting animals. Secondary pneumonia (further information available under Bovine Respiratory Disease) can develop with death ensuing quickly if untreated.
Clinical disease has an incubation period of around three weeks from ingestion, and can therefore occur a few days before larvae appear in the faeces. By the time clinical disease is present, the health of the cow or calf is already severely compromised, whatever treatment is then chosen. Because of the extensive damage that can be caused by worms in the lung tissues, this is a classic case of prevention being better than cure.
However, it is important to note that in contrast with other parasitic diseases, immunity to lungworm can develop relatively fast, achieving a steady state in which no clinical signs are observed (it is, after all, a poor parasite that kills its host). But without vaccination, such immunity can only develop if the cow or calf has received just enough natural challenge from the pasture in the past – neither too much, nor too little.
Unfortunately, the level of natural challenge depends on far too many factors to be regulated simply by farm management or worming routines.

Diagnosis
Diagnosis may be made on clinical grounds (signs of coughing, increased respiratory rate) and based on history, exposure status and seasonality. Adult and larvae may be seen on broncho-alveolar lavage samples. If lungworm are patent, then larvae may be found in the faeces of infected cattle when samples are analysed by the modified Baermann technique. Waiting on faecal larval counts using modified Baermann technique is risky as animals may develop pre-patent hoose (disease before larvae are passed in the faeces). There is a characteristic plasma eosinophilia though this is not diagnostic or uniformly present.
Control
Combining vaccination and worming is an effective means to prevent disease in cattle.
It is a commonly held belief that some wormers “allow natural immunity to develop” as a claim, in itself, to “create immunity” – something which of course only a vaccine or a (risky) natural challenge can do. Ultimately farmers must devise comprehensive ‘wholefarm’ strategies for parasite control, that are both cost-effective and sufficiently protective in the longer term, in conjunction with their local veterinary practitioner.
A control plan could include the following aspects?
1. Vaccination of all cattle, then annual youngstock vaccination. For more information on the vaccine click here.
2. Vaccination of all incoming cattle
3. Control by management or rotational grazing. This is particularly difficult for D. viviparus control as immunity
does not develop in all of the cattle simultaneously and other helminths have different rotational grazing requirements for immunity development. In one study by Eysker (1992), it was found that rotational grazing of
calves in 6 small plots for one week allowed good immunity development against D. viviparus but did not protect the calves against gastrointestinal helminthiasis. Barger (1997) wrote about 3 strategies for worm control – preventive management (clean pasture or early season worming), evasive management (movement of cattle following early immunity development) and dilution (older immune cattle or different species on the pasture
concurrently).
4. De-worming strategies are also difficult to manage. Ideally cattle would graze until hoose developed, about 3 to 4 weeks later. Only sick or coughing animals would be treated with an anthelminthic prior to economic loss – weight loss etc. Other cattle would be treated when showing signs of infection, probably another 3 to 4 weeks later when the next wave of larvae had caused disease and stimulated adequate immunity. Not only is this not very practical but it may select for anthelmintic resistance. This is more likely to occur if animals are treated very frequently
or with an anthelminthic that does not kill all of the worms. Pulse wormers can be useful for some calves in the group but may not allow sufficient immunity to develop in all of them leading to disease in subsequent years. Some pulse wormers are better than others. Consult your local veterinary practitioner for details.
More information on vaccination may be obtained by viewing the Huskvac technical bulletin.
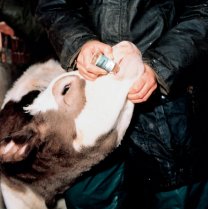
Vaccination
Only one vaccine is available for lungworm. Known as Bovilis Huskvac, it is a live vaccine containing irradiated larvae for oral administration. For more information on Bovilis Huskvac, click here .
